HFFR
HFFR
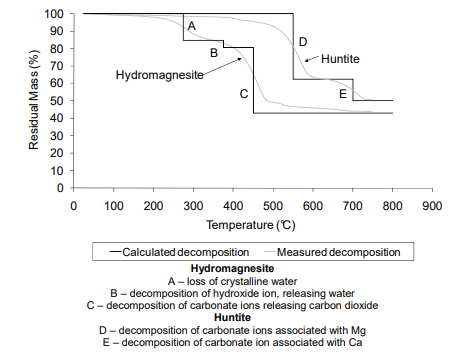
Naturally occurring mixtures of hydromagnesite and huntite are important industrial minerals.
Their endothermic decomposition over a specific temperature range, releasing water and carbon dioxide, has lead to such mixtures being successfully used as fire retardants, often replacing aluminium hydroxide or magnesium hydroxide.
The thermal decomposition of huntite has been characterised and is relatively simple. However, the thermal decomposition mechanism of hydromagnesite is sensitive to many factors including rate of heating and the composition of the atmosphere. The partial pressure of carbon dioxide significantly effects the decomposition mechanism of hydromagnesite causing magnesium carbonate to crystallise and decompose at a higher temperature instead of decomposing directly to magnesium oxide.
Hydromagnesite has to decompose endothermically releasing water and carbon dioxide over a temperature range of approximately 220°C to 550°C.
Mg5(CO3)4(OH)2.4H2O → 5MgO + 4CO2 + 5H2O
This endothermic decomposition and release of inert gases gives hydromagnesite its fire retardant properties.
Huntite also decomposes endothermically releasing carbon dioxide over a temperature range of approximately 450°C to 800°C.
Mg3Ca(CO3)4 → 3MgO + CaO + 4CO2
While this is too hot to coincide with major polymer decomposition and fuel production stages occurring around ignition, its platy morphology reinforces the formation of a thermally protective barrier layer which may reduce the rate of burning.
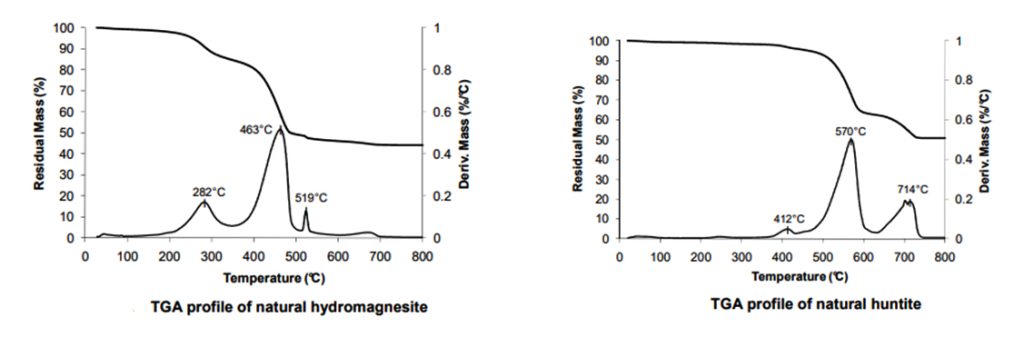
Decomposition of hydromagnesite begins to occur at about 220°C and progresses through two major mass losses.
Huntite begins to decompose at about 450°C and again progresses through two major mass losses. The mixture of hydromagnesite and huntite provides a broad decomposition range starting at about 220°C and being complete by about 740°C. During these decomposition reactions approximately 54% of the original mass of the mixture of hydromagnesite and huntite is released as carbon dioxide and water.
Figure shows differential scanning calorimetry (DSC) measurement, made by the present authors, using a Rheometric Scientifics DSC1500, under a nitrogen atmosphere, with a heating rate of 10°C min -1.
A mixture of hydromagnesite and huntite shows that each of the decompositions measured by TGA is associated with an endotherm.
The total heat of decomposition of the mixture is approximately -990 Jg-1. This figure will vary depending on the ratio of the two minerals.